By combining a Blow-Fill-Seal Container with a pen needle hub, we’re making drug delivery more*…

What if the key to transforming drug delivery wasn’t to make it more complex, but to make it simpler? Welcome to Apiject. Our platform makes it possible for injections everywhere to be delivered with the safety and high performance of a prefilled injector.*

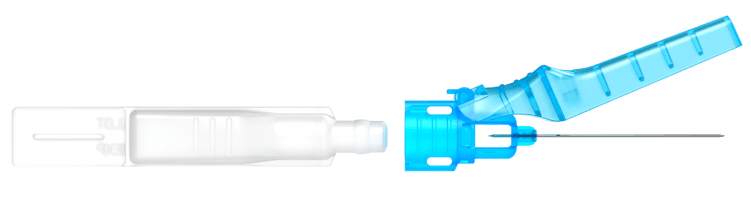






By combining a Blow-Fill-Seal Container with a pen needle hub, we’re making drug delivery more*…

At the core of the Apiject Platform is a Blow-Fill-Seal container adapted for injection use. The single dose container is aseptically filled and combined with a proprietary pen needle hub similar to those used for insulin. We use only two primary materials – medical grade plastic resin and stainless steel rolls – ensuring a simple and reliable supply chain.
Explore the world and technology of Apiject. Read our latest company news, listen to our experts, and watch our team’s progress as we strive to make all injections prefilled.