Blow-Fill-Seal Temperature Management
INTRODUCTION TO BLOW-FILL-SEAL (BFS) TEMPERATURE CONTROL
Over the last 30 years, technology and process advancements have allowed manufacturers to fill-finish a wider range of sterile liquids using BFS, including many biologics and vaccines, small molecules, and other pharmaceuticals.
At ApiJect, our engineers have taken these filling enhancements even further by developing proprietary temperature management methods that can potentially enable many temperature sensitive biologics and vaccines that are not possible on standard BFS lines. Our experts’ deep experience with fill-finish, combined with our technology, may be able to benefit your drug product development programs.
UNDERSTANDING TEMPERATURE IN BFS
The BFS process is divided into five main stages:
Extruding: Heated, liquefied resin is extruded to form a parison within the BFS mold.
Blowing: Sterile air is flushed through the parison as the mold closes and the container is formed.
Filling: Using a time/pressure fill system, an exact amount of drug product is dispensed into the container.
Sealing: The top of the mold comes together, forming an aseptically sealed container.
Demolding: The mold is opened and the filled container(s) are released.
Each BFS Container is produced in a strip, with a continuous sheet being produced that is then punched into single strips and eventually separated into cards.
IMPROVED TEMPERATURE CONTROL MECHANISMS AND PROCESSES
Our BFS manufacturing experts employ a variety of techniques to consistently manage the drug product’s temperature throughout the fill-finish process, including:
Mold Design: The shape of the BFS container has a significant impact on managing the temperature of the drug product. For example, we have designed the BFS Container in our prefilled ApiJect Prefilled Injector* to minimize the surface area that the drug product contacts, which reduces the heat transfer from the container walls to the drug product.
Wall Thickness: Our engineers have designed the BFS containers to use a minimal amount of plastic in order to expedite the cooling process, while still maintaining the integrity of the vial.
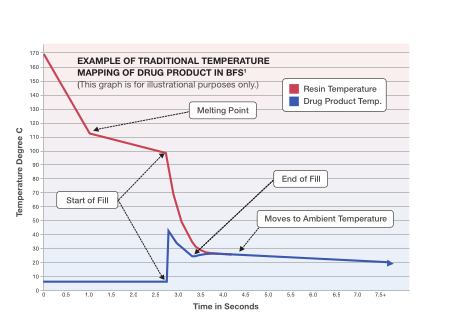
Pre-Fill Cooling: We employ a variety of proprietary methods to chill the drug product and molds until the very moment the drug product is filled in the BFS container. The chart above is an abstract from a general mathematical model1 that estimates the temperature of the container and a 0.6mL fill drug
product as it goes through the BFS cycle on a Rommelag® bp434 machine. ApiJect’s BFS experts have extensive experience using various methods of control to minimize high temperature exposure.
Post-Fill Cooling: After filling, container separation, and leak detection are complete, our engineers have developed processes to rapidly chill the finished units prior to packaging.
*The prefilled ApiJect Injector has not been approved by the FDA or other regulatory authorities for distribution.
1
The chart has been abstracted from an original published report by Jeff Price
(then with Vital Pharma, Inc.) in 1998, and has been updated by the author for representative purposes only.
FOR MORE INFORMATION:
Blow-Fill-Seal: visit https://bit.ly/3l27Qwy
Expert Content Library: visit https://bit.ly/3IZrEZm
Manufacturing: visit https://bit.ly/3YwKiOc
Company Profile: visit https://bit.ly/3ZZya9F
Email: [email protected]

