Partnering for Success
APIJECT TECHNOLOGY — REDEFINING INJECTABLE DRUG DELIVERY
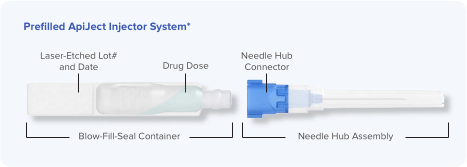
At ApiJect, our experts design proprietary injection systems that allow partners to deliver their drug products using cost-effective Blow-Fill-Seal (BFS) manufacturing technology. Our first designed device is the Prefilled ApiJect Injector*: a proprietary, sterile, unit-dose injection system that is market differentiated, commercially scalable, and provides the safety, convenience, and time savings of a prefilled format to the end user.
The standard design can be seen in the figure below, which illustrates two primary components: a BFS container (low-density polyethylene) with the drug product and an attachable needle hub with needle shield.
A PLATFORM TO PROVIDE DRUG DELIVERY SOLUTIONS
As parenterals continue to grow, more companies are looking for solutions that deliver the benefits of prefilled formats but with the manufacturing efficiencies of glass filling. That is why we created the ApiJect Platform: a design and manufacturing process that merges BFS and plastic components to create single-dose, prefilled parenterals customized for your drug product.
While a 0.5mL Prefilled ApiJect Injector*, was created for vaccines and other smaller-dose products, the ApiJect Platform is designed to serve a very wide range of parenterals for all markets. Our scope includes all major injection routes of intramuscular, subcutaneous, intradermal and intravenous, as well as traditional BFS routes of oral, otic, optic, and inhalation. In addition, BFS can handle a wide range of fill volumes, including injectable doses ranging from 0.25 mL to 5.0 mL
*The Prefilled ApiJect Injector has not been cleared by the FDA or other regulatory authorities.
EXPANDING BFS FOR INJECTABLES
Over the last 20 years, BFS engineers have made strong improvements that allow BFS to serve a wider range of sterile liquid pharmaceuticals. ApiJect experts have advanced this process much further with our temperature management technology. This proprietary process enables BFS to potentially serve a wide range of heat-labile injectables, including biologics, in addition to emulsions, suspensions, and other challenging formulations.
MARKET CONSIDERATIONS
With the intense global growth of sterile injectables over the next ten years, it is the right time to reconsider the best process to meet the increased demand of the medical community and patients.
BFS offers several advantages to manufacturers, including:
- Advanced aseptic process
- High-speed fill rates
- Low cost of goods
- Primary package comprised of one raw material
- Efficient production processes
In addition, the flexibility of BFS output enables a single line to produce up to 25,000 units of drug product per hour.
DESIGN, PROTOTYPING, & TESTING
At the Technology and Development Center, our device and BFS experts leverage the ApiJect Platform to design the right drug delivery system for your drug product.

APIJECT AS YOUR PREFERRED PARTNER
ApiJect continues to invest in R&D to develop innovative ways of enhancing device performance for the best patient experience as well as a focus on modular BFS manufacturing to simplify commercial technology transfer.
Whether you have a branded or generic pharmaceutical product, ApiJect has flexible arrangements to work with your team from select development services to full-scale commercial production through our growing partner network in the United States and Europe.
As a first step, speak with our business leaders about conducting a feasibility study with your drug product.
FOR MORE INFORMATION:
Blow-Fill-Seal
Expert Content Library
Manufacturing
Company Profile
Email: [email protected]
ApiJect Systems, Corp.
2 High Ridge Park, Stamford, CT 06905
Phone: (203) 998-6176
Email: [email protected].