Global Manufacturing Supply Chain Resiliency
COVID-19 has shown that pharma companies need to make their complex and inefficient global supply chains more resilient. With high-value therapeutics at stake, having a flexible, resilient, and robust manufacturing supply chain is more important than ever. At ApiJect, we have created a compact and flexible supply chain that can be almost entirely domestic in major markets. This is because we rely on two robust manufacturing technologies to make our platform: Blow-Fill-Seal (BFS) aseptic filling and plastic injection molding. It was one of the primary reasons the U.S. Government began supporting ApiJect R&D for potentially faster bio-emergency response even prior to COVID-19.
RELIABILITY OF BFS MANUFACTURING
The reliability and scale of BFS aseptic manufacturing helps ensure a more flexible and resilient supply chain – even during a global pandemic.
Recognized by the industry and regulators as an advanced aseptic process, BFS delivers a high-quality product at any manufacturing scale. Advantages of BFS include:
- Production lines require minimal human intervention.
- Depending on mold installation requirements, a BFS machine could be turned over within one day
- Larger-scale BFS machine models can aseptically fill up to 15M 0.5mL units per month.
- Very low particle generation because the filling process is done in a contained ISO-5 environment.
SIMPLIFIED MATERIAL PROCUREMENT
Compared to more traditional injection fill-finish formats, the ApiJect Platform, which creates BFS-based prefilled injection devices, has designed simplicity and
reliability into its material procurement and production supply chain.
BFS and the ApiJect Platform require only two primary materials to be procured: pharmaceutical-grade resins (LDPE and PP) and cannula.
- Both materials can typically be sourced in all major markets and reliably produced in sufficient scale.
- Needles and resin potentially can be stockpiled at the manufacturing facilities, ensuring localized supply.
- Made of low-density polyethylene, BFS containers have very limited delamination issues.
- Materials used in the secondary packaging process, such as a drug label, foil pouch, and boxing, are generally also available in all major markets.
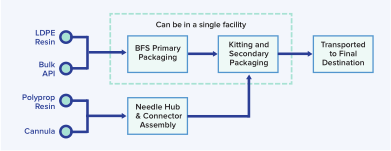
HIGHLY COMPACT END-TO-END SUPPLY CHAIN
As opposed to traditional glass filling lines that have many components and involve multiple facilities, the supply chain that supports the BFS process and the ApiJect Platform can require as few as two facilities: one for BFS fill-finish and another for attachable component manufacturing.
Advantages of this compact supply chain include:
- Needle Hub manufacturing for Prefilled ApiJect Injectors is done by Tae-Chang Industrial of South Korea, the world’s second largest cannula supplier by volume.
- A BFS filling line only requires approx. 500 sq. ft. of ISO-7 space.
- ApiJect pre-set modular manufacturing units fulfill response for local, regional and international sterile manufacturing needs.
- BFS containers are resistant to breakage or shattering, making them a very robust option for distribution and warehousing in all markets.
- Kitting of the BFS containers and attachable components can be done in the same facility as the BFS manufacturing (today it is done in a separate facility).
MANUFACTURING EFFICIENCY
Advantages of BFS include:
- The light weight and breakage-resistant nature of resin containers versus standard glass vials have the potential for reduced transportation and associated wastage.
- In addition, the ApiJect Platform is designed to reduce downstream complications, including space efficiency of cold chain storage, breakage, and high overfill, as compared to traditional single-dose formats.
- Once a mold is installed and the line is qualified, a BFS machine can scale up reliably and quickly.
FLEXIBILITY FOR CONTINUOUS INNOVATION
One reason the ApiJect Platform relies on BFS and its supply chain is its ability to allow for innovative container and drug delivery design.
By modifying the mold design, ApiJect engineers are able to rapidly test the performances of different BFS container shapes. This commitment to R&D help ensure pharma customers will be served with optimal performance of any licensed ApiJect technology and device design.
